Jay S. Chandra, PhD
STRATEGIC ADVISOR IN MEDICAL AND SCIENTIFIC AFFAIRS, CLINICAL DEVELOPMENT & BUSINESS STRATEGY
Helping early-stage biotech teams design smarter trials, navigate complexity, and drive scientific clarity.
About Me
I’I’m a Biomedical scientist and Biotech strategist with over 15 years of experience in leading and managing global clinical trials across medical and scientific affairs as well as clinical development. This includes multiple therapeutic area expertise – Neuroscience, Medical devices, Musculoskeletal disorders, Oncology, Ophthalmology, Gastroenterology among others.
I’ve advised top leadership, C-suite at major pharmaceutical companies, CROs, and startup teams on high-impact studies, strategic pivots, and scientific communication that builds confidence.
People work with me not just because I’m technically sound — but because I combine sharp strategy with calm, clear communication that helps teams cut through complexity and regain momentum when things stall. Whether it’s untangling a misaligned study or guiding tough clinical decisions, I partner with teams to bring clarity to complex science.

Early-Stage Biotech Consulting
Clinical Strategy & Trial Optimization
I help biotech teams shape smarter trials and solve complex clinical challenges — from protocol refinement to vendor troubleshooting and milestone recovery. As a strong liaison between scientific leaders and operational teams, I bridge communication gaps and ensure alignment across functions. Whether you’re launching early-phase studies or rescuing a stalled one, I bring calm clarity and strategic foresight to move things forward.
Scientific Positioning & business development
From KOL alignment to cross-functional messaging, I help teams communicate science with precision and impact. I guide what to say, how to say it, and who needs to hear it. I contribute creative scientific input to marketing teams, helping translate complex data into compelling, strategic content.
Innovation Due Diligence & Expert Opinion
I support founders, and R&D teams with deep scientific vetting of assets, IP, and trial designs. My input bridges the gap between invention and implementation — offering technical diligence, clinical feasibility insight, and clear-eyed perspective on risk, readiness, and real-world potential.
Consulting Focus Areas
Clinical Strategy & Trial Optimization
Strategic Advisory for Biotech Leadership
- Thought leader for CSOs, CEOs, founders, and clinical executives
- Scientific input during pivots, scale-ups, and high-stakes decisions
- Strategic clarity for cross-functional alignment and funding narratives
- Bridging vision with execution — calm, credible, and actionable
- Clinical Development & Trial Design
Clinical Development & Trial Design
- Imaging and endpoint optimization
- Scientific oversight for early-phase or high-complexity studies
- CRO/vendor evaluation and mid-study remediation
- Root-cause analysis for delays and study misalignment
- Scientific conflict resolution with CROs and internal teams
Clinical Strategy & Trial Optimization
Strategic Advisory for Biotech Leadership
- Thought leader for CSOs, CEOs, founders, and clinical executives
- Scientific input during pivots, scale-ups, and high-stakes decisions
- Strategic clarity for cross-functional alignment and funding narratives
- Bridging vision with execution — calm, credible, and actionable
- Clinical Development & Trial Design
Clinical Development & Trial Design
- Imaging and endpoint optimization
- Scientific oversight for early-phase or high-complexity studies
- CRO/vendor evaluation and mid-study remediation
- Root-cause analysis for delays and study misalignment
- Scientific conflict resolution with CROs and internal teams
Clinical Strategy & Trial Optimization
Strategic Advisory for Biotech Leadership
- Thought leader for CSOs, CEOs, founders, and clinical executives
- Scientific input during pivots, scale-ups, and high-stakes decisions
- Strategic clarity for cross-functional alignment and funding narratives
- Bridging vision with execution — calm, credible, and actionable
- Clinical Development & Trial Design
Clinical Development & Trial Design
- Imaging and endpoint optimization
- Scientific oversight for early-phase or high-complexity studies
- CRO/vendor evaluation and mid-study remediation
- Root-cause analysis for delays and study misalignment
- Scientific conflict resolution with CROs and internal teams
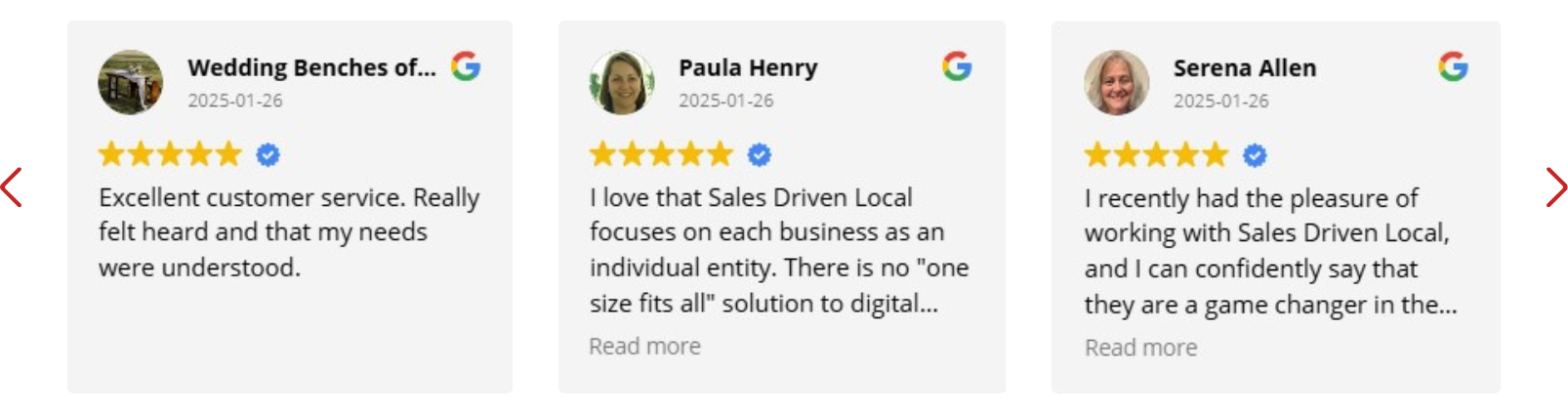
What People are Saying
Don’t Let Your Business Blend In or Miss Out on Local Sales
With Chesapeake Bay Solutions, you can be the brand that locals trust, remember, and recommend.
How do we do this?

Digital Marketing Strategy

Communication Automation

Logos and Branding

Websites

Funnels

Graphic Design

Search Engine Optimization

Email Marketing Strategy and Content

Copywriting

Social media content